The World is experiencing increasingly severe extreme weather events – droughts, bush fires and floods due to climate change. Achieving net-zero emission of carbon dioxide (CO2) by 2050 will be critical to limit the dangerous impacts of climate change.
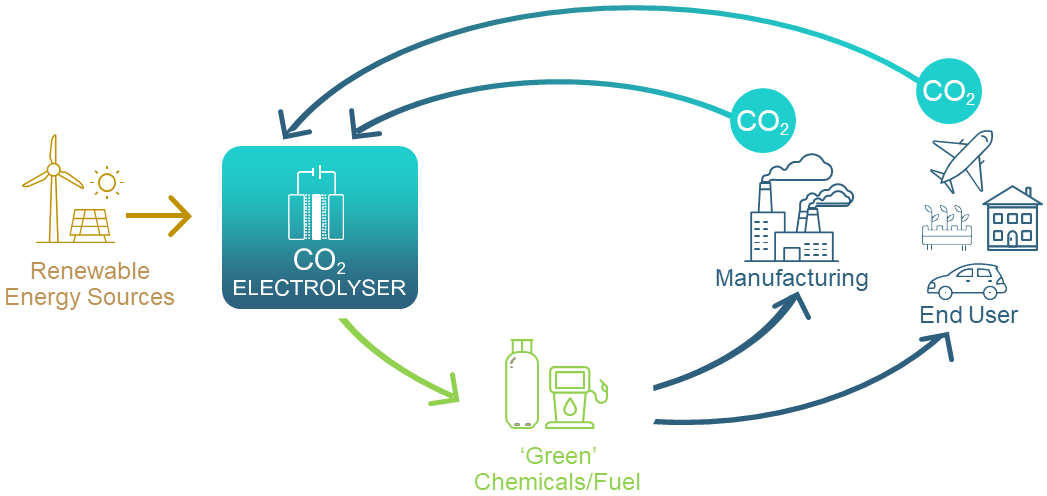
GETCO2 will assist Australia to meet national and international net-zero obligations. The transformation of CO2 to value-added products will underpin a circular carbon economy, helping to reshape Australia’s energy and resource export industries for long-term resilience and growth.
CO2 utilisation is essential for decarbonisation
Carbon capture and transformation use CO2 as a resource for generating valuable, carbon-containing products, such as fuels, fertilisers and building blocks for commodity chemicals. Currently, our economy relies heavily on fossil fuels to supply carbon for manufacturing these products, whereas CO2 can be sourced from industrial emissions, biogas, directly from the atmosphere, or even from the oceans.
Electrochemical conversion of CO2 is commercially attractive
Potentially, many commercially important chemicals such as formaldehyde, methanol and ethylene can be produced electrochemically from CO2 and H2O. Importantly, electrochemical CO2 conversion can be powered by renewable electricity. Australia’s vast potential for low-cost, renewable energy generation, enables the opportunity to create new ‘green’ technologies and develop new manufacturing industries.
Principles of CO2 electrolysis
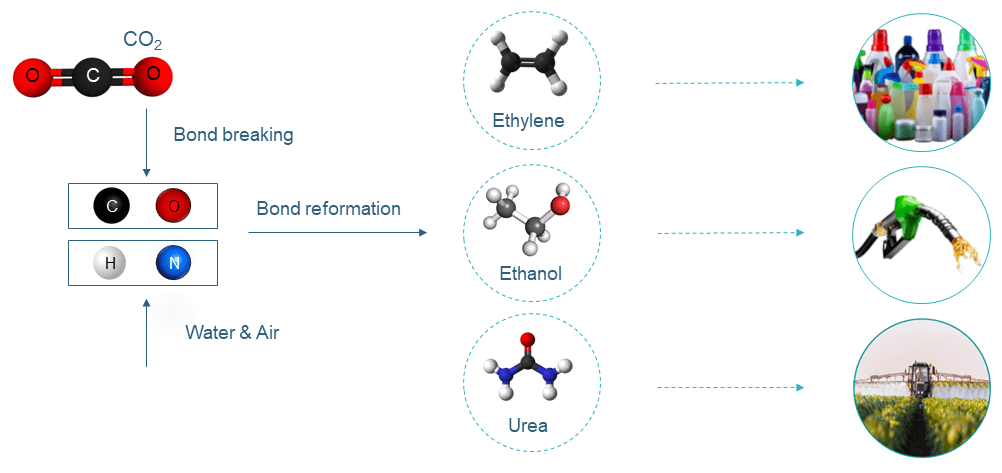
CO2 is comprised of one carbon atom bonded with two oxygen atoms. CO2 is a very stable molecule, and energy is needed to break the molecular bonds. Under certain conditions, the atoms can then be bonded with other molecules such as C, O, H, N to form desired chemicals.
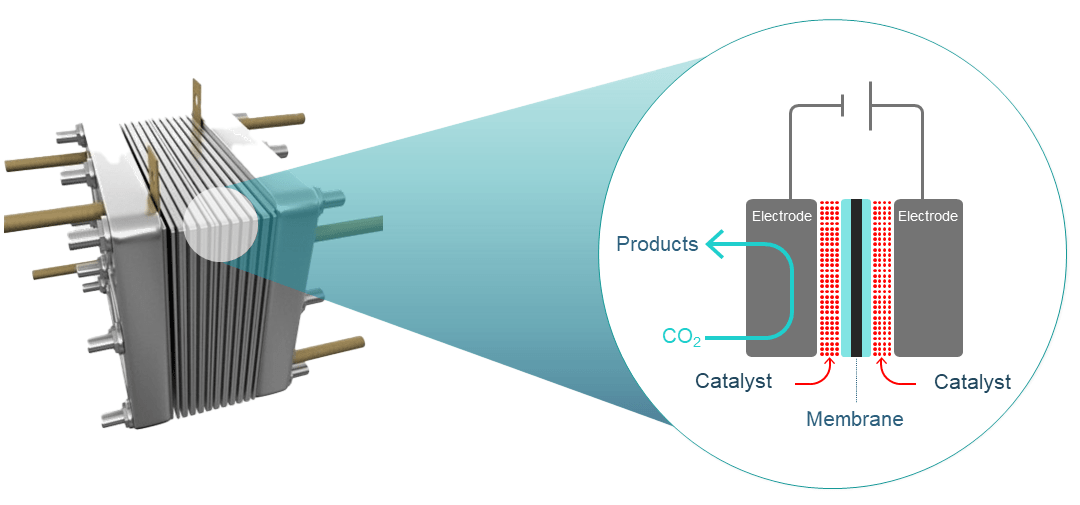
A CO2 electrolyser comprises a cathode (negatively charged electrode) and an anode (positively charged electrode) in contact with electrolytes and separated by an ion-exchange membrane.
The electrodes are coated with different catalysts to lower the energy required to break chemical bonds and promote selective bond formation to deliver desired products.
Typically, CO2 conversion occurs at the cathode, while oxygen is evolved at the anode.
The frontier
CO2 utilisation is a fledgling industry, but globally is expected to reach up to US$1 trillion by 2030.
Three major scientific hurdles challenge the viability of the technology:
- low carbon and energy efficiencies
- low selectivity for producing higher-value chemicals
- poor durability of equipment
For CO2 electrolysis to be economically viable, the technology must be energy-efficient, durable over prolonged operation, cost-effective and tuneable to deliver targeted value-added products.
GETCO2 will forge technological breakthroughs to accelerate reaction rates, vastly improve product selectivity and significantly extend operating life for electrochemical CO2 conversion.